Advances in medical therapies and diagnostics result from integrated efforts of multidisciplinary teams that take an idea from concept to market. Ideas are generated by health care workers from a wide spectrum of professional and educational backgrounds. Assessment of the potential applicability (market analysis) and patentability of ideas leads to assignment of value for the purpose of procuring funding. Engineers trained in the sciences of design, prototyping, and manufacturing create products according to rigorous quality standards to ensure safety, functionality and reliability. Preclinical (bench and animal) testing establishes basic aspects of safety, functionality, and efficacy, while clinical testing is performed to determine clinical utility (i.e., how the product benefits the patient). Such testing relies on partnerships between sponsors (industry), physicians, hospitals, and volunteers (patients) involving a host of real and implied legal, ethical and moral responsibilities. Final approvals from regulatory bodies take into account results from the entire history of the product’s development and evaluation. In the current social, economic, and political environments, development costs and the costs to deliver new therapies and diagnostics are of paramount interest and importance. Ideally, oversight of the entire process by an experienced management team ensures seamless integration of the appropriate teams to achieve the end result of an approved, marketable product.
The entire process of taking an idea from concept to market is called translational research. In the past, translational research has been defined simply as application of findings from basic sciences to enhance human health and well-being. However, with recognition of the complexity of the process, translational research can itself be considered a discipline that is subject to advancement through scientific methods based on the cycle of hypothesis generation and testing. Accordingly, we can propose to define translational research more broadly as “a field of science that aims to develop concepts for medical therapies or diagnostic tests from knowledge gained through basic or clinical research and establishes their safety and efficacy through cost-effective processes.” From a practical perspective, declaring translational research as a field of science in this manner establishes the concept that the process of generating new therapies and diagnostics can be improved so that available funding will more likely lead to successful launch of products at lower costs to patients.
Traditional educational curricula are well established in teaching the individual components of translational research, particularly when it comes to medical sciences and engineering. However, exposure to topics such as market analysis, intellectual property, venture capital funding, quality systems, and regulatory requirements, which are the “glue” of the translational process, are traditionally obtained through specialty education, certification courses, seminars, or on-the-job training. It is not suggested that every individual interested in translational research need become expert in all these fields. However, lack of high-level understanding of what it really takes to create a medical therapy or diagnostic test leads to inefficient use of time and money. Consequently, many brilliant ideas never make it to the clinic or, conversely, significant resources are spent on ideas with little chance of success.
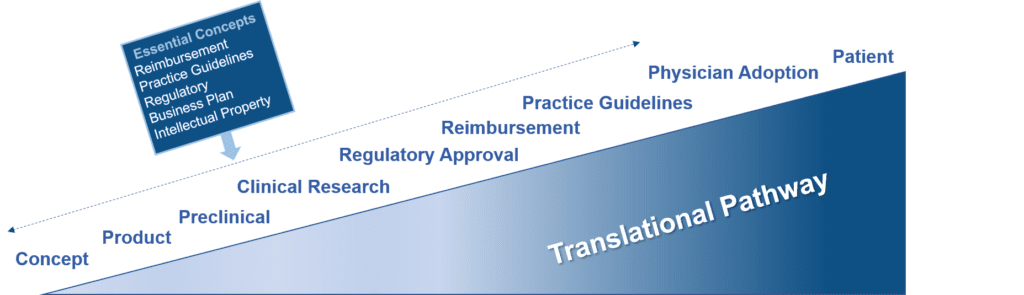
While there is a recognized need to develop formalized curricula for teaching the foundations of translational research within academic institutions, such programs are currently in their infancy. The goal of this document is to bring together elements of each step of the process (Figure 1) and to provide specific examples where this process has been successfully applied.
Figure 1. Steps in the Translational Research Pathway