The difference in radial expansion with an early replacement valve is demonstrated in this cadaveric heart. (Used with permission from Stanton Rowe.)
Stanton Rowe
Alain Cribier, MD
Martin B. Leon, MD
The first rule of translational research must be to make something useful, or, said another way, make something that patients and/or physicians prefer. It can improve outcomes, be easier to use, or simply be more cost effective; any or all of these create value and utility.
In the aortic arena, translational research in 1999 was both daunting and easier. It was a formidable task in that the recognized standard of care was surgical aortic valve replacement, a long-perfected procedure with superb outcomes. Cardiothoracic surgeons published small series on treating the very elderly, stretching beyond the typical 70- to 80-year-old patient presenting with senile aortic stenosis (AS).
Given the established results, the need for any alternative therapy became questionable. Balloon aortic valvuloplasty, once considered a great hope for the small number of non-surgical patients postulated, had long since been relegated to palliative therapy either as bridge to surgery or for symptomatic relief for the truly non-surgical patient.
The pioneering efforts of Spyridon D. Moulopoulos, MD, and Dusan Pavcnik, MD, PhD (Figure 1 with images from 1980 and 1992 respectively), were left at the animal study phase and were not widely known as the first forays into transcatheter valve development. The fundamental and pioneering work of Henning Rud Andersen, MD, and his colleagues in Aarhus, Denmark, in the early 1990s was little known despite efforts at publication and licensing that fell far short of expectations (Figure 2). The world was not quite ready for transcatheter aortic valve replacement (TAVR), and the Andersen team was just too far ahead of the curve.
Figure 1. Valve Device Animal Studies
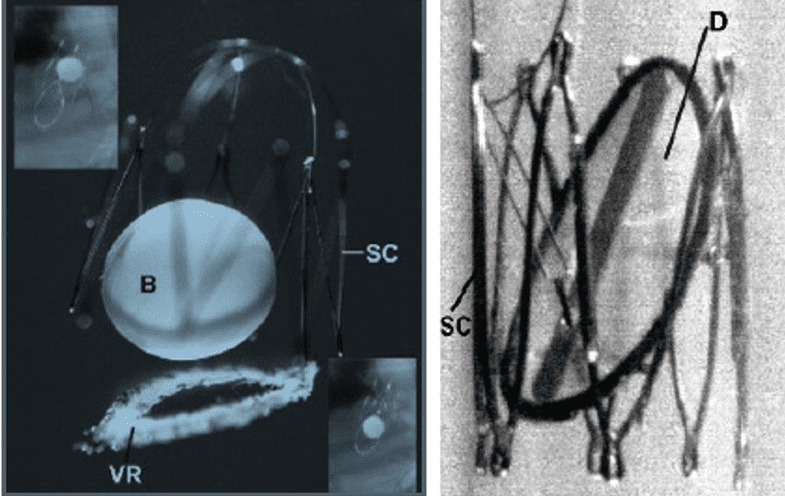
These early valves from Moulopoulos and Pavcnik did not progress to human studies and, at the time, were not acknowledged for their impact on transcatheter valve development. (Photos used with permission from Stanton Rowe.)
Andersen and his team described the first collapsible and expandable tissue valves. These were hand-made prototypes, in which they formed stents out of sternal wires on an internally developed jig and resected porcine valves from pig hearts purchased at the local butcher shop. The results, although not refined, were functional and illustrative of what was to come almost a decade later.
Figure 2. First Expandable Valves
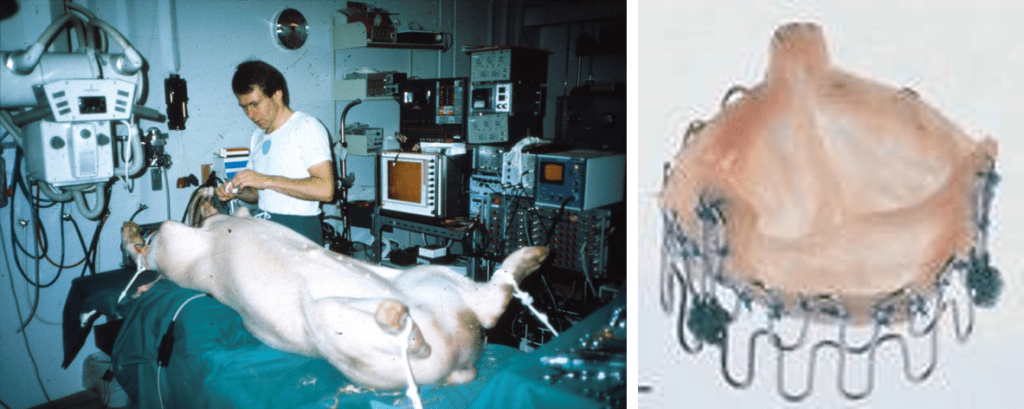
Henning Rud Andersen, MD, performs early animal studies with expandable tissue valves. (Used with permission from Stanton Rowe.)
Alain Cribier, MD, not knowing of Andersen’s work at the time, independently came to many of the same conclusions regarding the requirements, structure, and function of transcatheter aortic valves. His early observations added important insights into design and function and broke the rules of cardiothoracic surgery: Cribier proposed to leave the diseased valve in place and stent the leaflets out of flow field with a robust stent design, which would also carry the replacement valve.
This important hypothesis was tested in early studies in Rouen, France, when aortic Palmaz stents were deployed into cadaveric hearts to demonstrate the feasibility of this novel concept. The focus on radial strength of the expandable frame led the early developers in Percutaneous Valve Technologies (PVT), the company formed to develop and market TAVR, to conduct testing in the laboratory of Renu Virmani, MD, at the Armed Forces Institute of Pathology. In severely calcific fixed cadaveric hearts, expandable frames were tested, demonstrating excellent radial expansion, even in the most severe cases of AS (Figure 3).
Figure 3. Testing of Expandable Frames
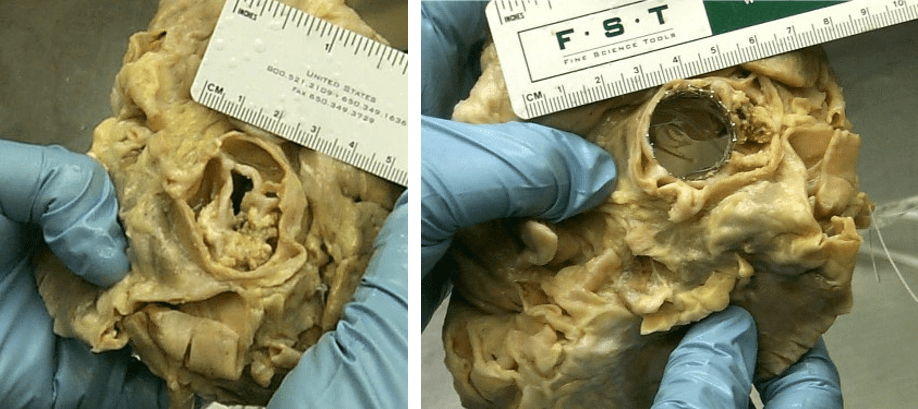
The difference in radial expansion with an early replacement valve is demonstrated in this cadaveric heart. (Used with permission from Stanton Rowe.)
Not surprisingly, there were many objections to the early concepts of TAVR, with cardiothoracic surgeons the source of most of the skepticism; the issues, however, were not disingenuous. The experience of surgeons doing aortic valve replacement naturally led one to conclude that stenting open aortic stenosis would be impossible. Spending every day removing aortic leaflets made largely of stone (calcium) would naturally make one incredulous that even with the best technology, this would not remotely be possible. Additionally, there was a natural apprehension focused on the potential for stroke, which remains today; but the original concern that every patient would experience a major stroke from such a procedure has certainly abated while fears regarding neurological complications following both TAVR and AVR remain active areas of investigation and debate.
Other concerns regarding valve durability, hemodynamics, prosthetic tissue damage, and coronary artery occlusion have largely subsided although understanding valve durability compared with surgical aortic valves continues to be an area of some debate.
Many skeptics were shocked to see the first report from Rouen from April 16, 2002, of the first aortic valve replacement without open heart surgery. A 57-year-old critically ill patient with many comorbidities, turned down for surgery by multiple teams, was successfully implanted with a PVT valve in a challenging trans-septal procedure necessitated by the lack of peripheral access. This historic case encouraged active development and investment in this field and has led to many varied designs and improvements both to products and procedures.
In this chapter, we will explore preclinical requirements and summarize current clinical status and standards of care, areas of debate and improvement, patent landscape, and future developments. Each chapter will be approached from the perspective of the translational researcher, a physician, or engineer who desires to improve the standard of care in TAVR and contribute to its future. (Please note: Many countries outside the United States continue to use the original term for the procedure, transcatheter aortic valve implantation or TAVI; for consistency’s sake, this book will use TAVR throughout.)