Inventors, Clinical and Basic Scientists, Interventional Cardiologists, Medical Students, Engineers, Industry, Regulators, Payers, and Investors
Inventors, Clinical and Basic Scientists, Interventional Cardiologists, Medical Students, Engineers, Industry, Regulators, Payers, and Investors
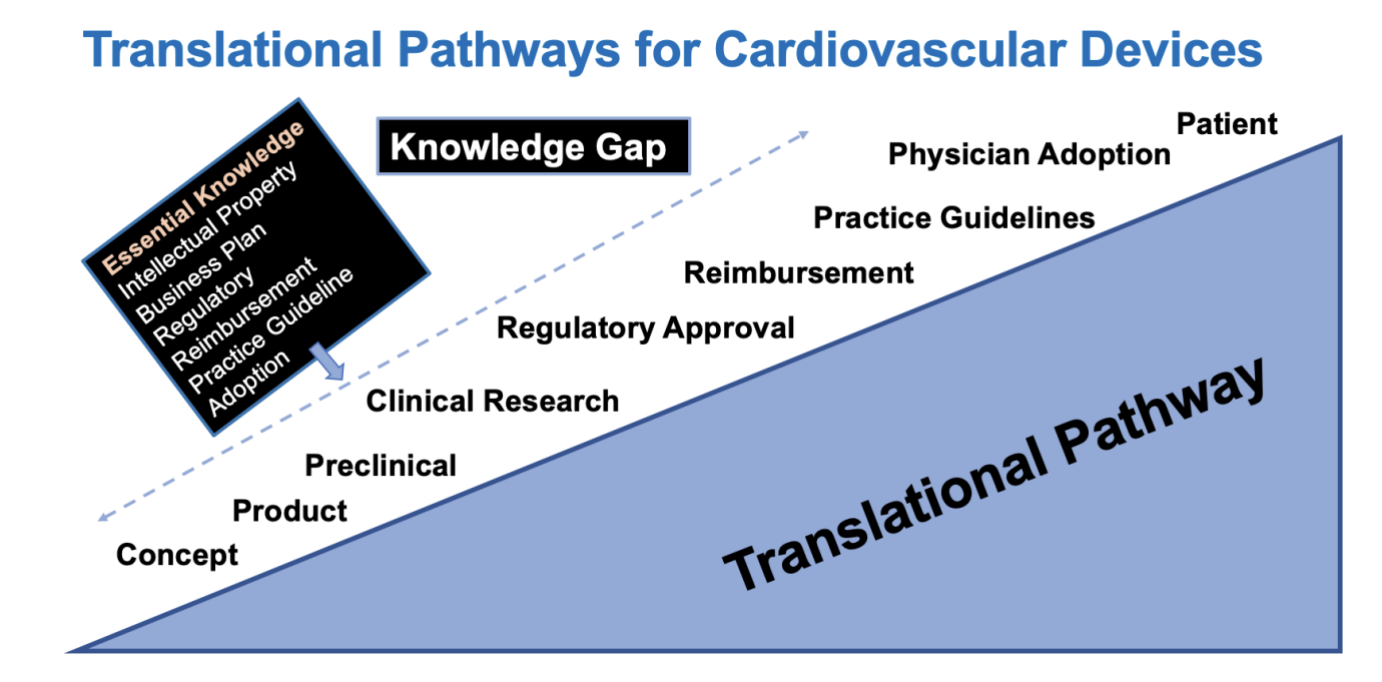
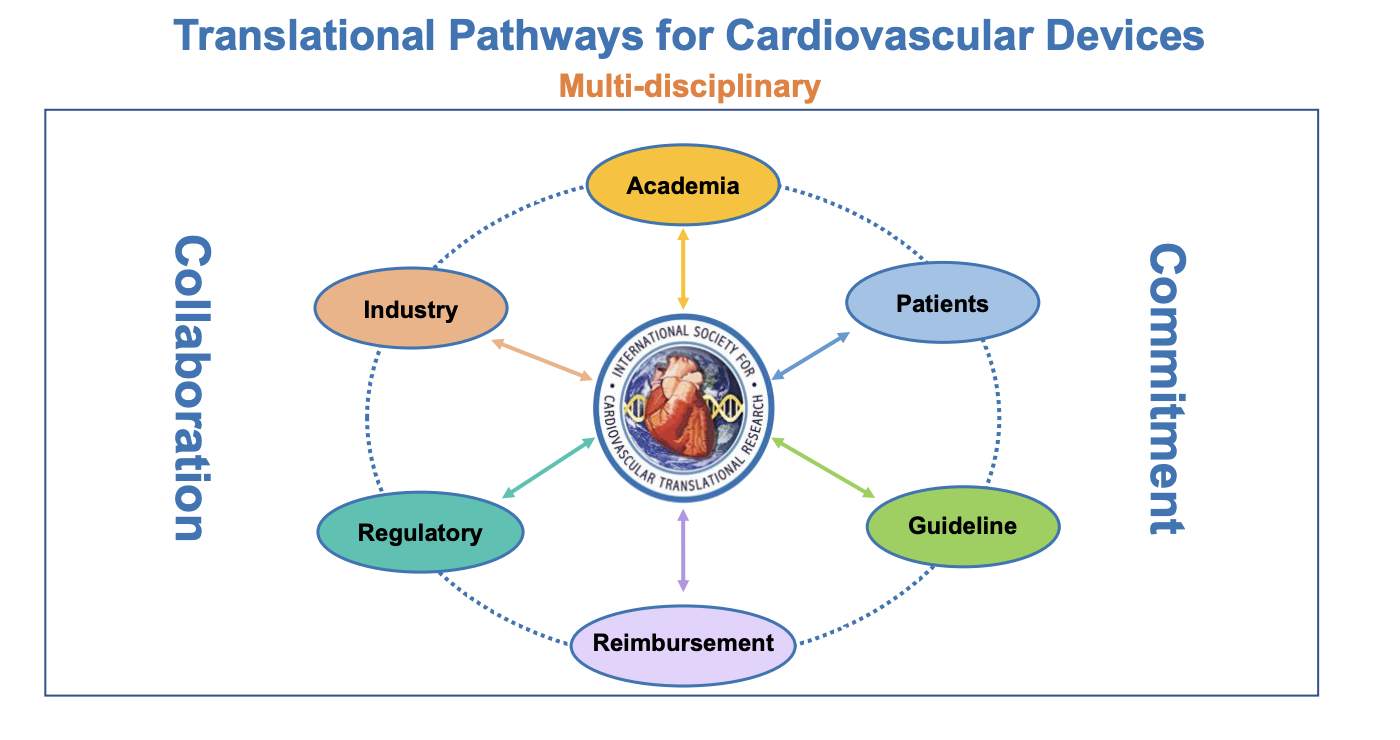
Choosing an Innovative Concept
Intellectual Property
Business Plan, Product Development, and Fundraising
Requirements for Medical Device Manufacturing & Iteration – FDA Point of View
Requirements for Medical Device Manufacturing & Iteration
Advanced Cardiac Anatomy – Application in Translational Research Tailored to Current and Future Technology
Large Animal Model for Heart Failure, Valvular Disease, Coronary Artery Disease, and Device Testing
Pre-Clinical Study Design & Endpoints for Device Evaluation – FDA Point of View
Pre-Clinical Study Design & Endpoints for Device Evaluation – Investigator Point of View
Early Feasibility Studies for Device Evaluation
Current Challenges & Future Direction for Human Early Feasibility Study for Device Evaluation – Industry Point of View
Basic in Statistics – Clinical Study Design for Translational Research
Basic Statistical Concepts
Sample Size and Power
Sensitivity and Specificity
Common Study Design
Phases of Translational Research
Statistics for Evaluation of Cardiovascular Diagnostic Devices
Pre-Clinical & Clinical Trial Design & Endpoints of Fast Track to Device Approval
Advanced Statistical Methods for Translational Research
Clinical Endpoints/Surrogate Endpoints
Regulatory Requirement for Marketing Approval
FDA Perspective on Transformative Regulatory Pathways & Device Innovation
Regulatory Review of Cardiovascular Diagnostic Devices – FDA Perspective
Regulatory Review of Cardiovascular Devices – European Regulatory Perspective
CMS Criteria for Reimbursement for Cardiovascular Innovation
Reimbursement for Diagnostic Devices
Practice Guideline Requirement for New Technology
Guideline Requirements for Diagnostic Devices
Adoption of Technology
Global Heart Health, Implications for Translational Research
Conflict of Interest and Product Development
The Patients Voice