Translational Pathway for Ventricular Assist Devices
Clinical Evaluations – Endpoints
Authors
David J. Farrar, PhD
Philip B. Adamson, MD, MSc
Keith Aaronson, MD
Introduction
Left ventricular assist devices (LVADs) have become the standard of care for patients with refractory end stage heart failure (HF) (1). These mechanical circulatory support (MCS) devices evolved from bulky pulsatile flow pumps from the 1980s and 1990s to small, durable continuous-flow LVADs (Figure 1) that can support blood flow in the body for many years with good quality of life (QOL) and reasonable adverse event profiles. Clinical trial endpoints have evolved as patient survival with MCS has significantly improved. Endpoints have moved beyond a focus on just patient survival, to composite endpoints that have become standard in modern clinical trials for durable MCS. For example, the recent MOMENTUM 3 (Multicenter Study of MagLev Technology in Patients Undergoing Mechanical Circulatory Support Therapy with HeartMate 3) clinical trial demonstrated significant improvement in the composite endpoint of survival free from disabling stroke or reoperation to replace or remove a malfunctioning device with the magnetically levitated HeartMate 3 LVAD compared to the axial flow HeartMate II (2,3).
Figure 1. Left Ventricular Assist Device Connected to the Heart and Aorta
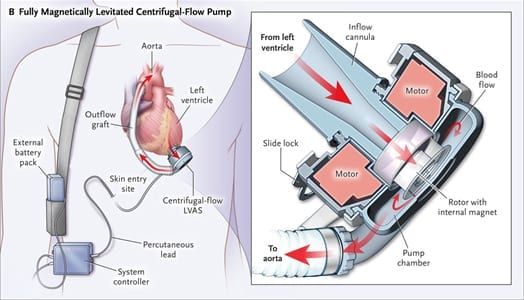
This example of a left ventricular assist device (LVAD) shows the HeartMate 3 centrifugal-flow pump receiving blood from the left ventricle with return via a graft anastomosed to the ascending aorta. The LVAD is powered via a percutaneous lead connected to an externally worn controller and two battery packs. LVAS = left ventricular assist system. (Reprinted with permission from Mehra MR, et al. N Engl J Med 2017;376:440-50.)
The design of clinical trials for objective assessment of safety and efficacy of implantable medical devices intended to treat terminal disease is challenging. Prospective, randomized, double-blind, placebo-controlled trials are the gold standard and provide the highest level of evidence when assessing new medical therapy. Device therapies, such as LVADs, pose a significant challenge to this standard paradigm as patients and investigators cannot be blinded to a non-implanted control patient. To overcome this dilemma, well-designed trials with carefully selected endpoints are paramount to define standard of care and obtain regulatory and reimbursement approval as well as to provide substantial evidence for expert-opinion guidelines.
Background of MCS Studies
Substantial progress was made over the last 4 decades in the design of implantable MCS systems, selection of appropriate recipients, and refinement of management protocols. During the 1970s, under the direction and funding of the U.S. Government’s Artificial Heart Program, and later under the Device and Technologies Branch of the National Heart, Lung, and Blood Institute (NHLBI), durable ventricular assist devices (VADs) underwent extensive research and development (4-6). Although heart transplantation became the treatment of choice in the 1980s, the severe shortage of heart donors and the expanding HF population intensified the need for durable MCS.
After the first successful bridge-to-transplant (BTT) case in 1984 with the a paracorporeal ventricular assist device (PVAD) (7), trials were carried out with pulsatile-flow devices assessing survival to transplant as the primary endpoint (8-10) or as a bridge to recovery (8). The nonrandomized BTT study using the HeartMate Implantable Pneumatic left ventricular assist device (IP-LVAD) was conducted, followed by studies of the HeartMate Vented Electric (VE) LVAD, which was more portable and allowed patients to be discharged from the hospital during support (11-13). In the VE LVAD BTT study, survival to transplant was significantly better than historical untreated control patients (71% vs. 33%), whereas the control patients had fewer serious adverse events (14). The positive survival-to-transplant outcomes in these BTT studies led to the REMATCH (Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure) trial, which was the first randomized controlled trial comparing a durable LVAD to medical therapy, and it was the first trial to study LVAD therapy for lifetime destination therapy (DT) (15). The 1-year survival rates in this 2001 publication were 52% in the LVAD group and 25% in the medical therapy group (p = 0.002); the 2-year survival rates were 23% and 8% (p = 0.09), respectively.
Clinical studies evaluating small, durable continuous-flow LVADs were initiated in the early 2000s(16-18), leading to U.S. Food and Drug Administration (FDA) approvals for the BTT (19) and DT (20) indications. These novel devices differed from the prior generation of pulsatile devices; they were small, highly durable, and arterial blood flow was continuous throughout systole and diastole. Clinical outcomes were enhanced by the reduction of serious adverse events and the lack of mechanical failures with the implanted pumps (20-22). Early studies demonstrated that continuous arterial blood flow provided adequate systemic perfusion as evidenced by improved or stable end-organ function (23-25). Following implantation, common adverse events included gastrointestinal bleeding, device infection, and stroke, which resulted in rehospitalizations and contributed to the cost of the therapy (26). As survival improved along with improved device designs, clinical trials began to assess post-operative and longer-term adverse events as components of composite endpoints to more fully assess patient risk-benefit relationships.
Clinical Trial Endpoints
Endpoints in clinical trials with durable LVADs typically include survival, adverse events, functional status, and QOL over time periods that range from 6 months to 2 years after implantation (Table 1).
Table 1. Key Endpoints in LVAD Clinical Trials
| Primary endpoint
|
|
| Survival |
|
| Adverse events
|
|
| Functional status |
|
| Health-related quality of life |
|
EuroQol = European Quality of Life; KCCQ = Kansas City Cardiomyopathy Questionnaire; LVAD = left ventricular assist device; MLHFQ = Minnesota Living with Heart Failure Questionnaire; NYHA = New York Heart Association; RVAD = right ventricular assist device.
Survival
Actuarial survival by the Kaplan-Meier method is the universal assessment of survival, with log-rank analysis for comparison between groups. Patients are censored from the analysis at transplantation or explantation of the device following cardiac recovery. Competing outcomes analyses are also common, showing the percentages of patients who receive transplant, have explantation for recovery, have died during LVAD support, or are still ongoing support throughout the trial period.
Adverse Events
MCS clinical trials assess the occurrence and severity of adverse events from the time of implantation. All recent MCS clinical trials have typically used definitions of adverse events based on those from the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) registry, which have changed slightly over the years (27). Adverse events are presented as the percentage of patients who had the event and/or are presented as event rates per patient-year (prevalence) or per 100 patient-months (incidence), and often with Kaplan-Meier curves showing time to first event. However, recurrent event analyses should be the standard methodology to evaluate adverse events with the possibility of multiple events during follow-up. Neurological events, cerebrovascular accidents, and gastrointestinal bleeding are examples of multiple adverse events during either short- or long-term follow-up trials.
Functional Status
The patient’s functional status is assessed by the 6-minute walk test (6MWT) and New York Heart Association (NYHA) functional class. The 6MWT measures the distance a patient can walk in 6 min – it is objective, reproducible, and easy to conduct. The NYHA functional class uses physical limitations and shortness of breath to categorize patients on a scale of 1 to 4. LVAD trials have shown significant improvement in 6MWT distances, and approximately 80% of patients reach NYHA class I or II compared to 0% before implant (3,28).
Health-related Quality of Life (HR-QOL)
Clinical trial instruments to provide subjective overall assessment of an individual’s happiness, satisfaction, and contentment with life include the European Quality of Life (EuroQol) EQ-5D-5L questionnaire (29), the Kansas City Cardiomyopathy Questionnaire (KCCQ) (30), and the Minnesota Living With Heart Failure Questionnaire (MLHFQ) (31). The KCCQ is acceptable for FDA studies, and the EQ-5D-5L was adopted by the INTERMACS registry. The EQ-5D-5L is a standardized, patient self-administered tool that assesses patient mobility, self-care, usual activities, anxiety/depression, and pain/discomfort on a five-point scale. The KCCQ is a self-administered questionnaire addressing the patient’s physical function, symptomatology, social function, knowledge, and QOL. LVAD trials typically find significant improvements in each HR-QOL instrument following implant in all of the measures. Patient-reported outcomes assessing QOL are now recognized as potential non-mortal primary endpoints in HF trials, but, as they tend to be subjective, these metrics are usually assessed as a part of composite or secondary endpoints. There are efforts to develop improved QOL instruments that reflect the challenges patients have specific to a life with long-term LVAD support. Disease specificity is important and the ongoing SUSTAIN-IT (Sustaining Quality of Life of the Aged: Heart Transplant or Mechanical Support) study (NCT02568930) is designed to compare HR-QOL, symptoms, thinking, and adverse events between LVAD and transplant patient groups.
Primary Endpoints
The primary and secondary endpoints of the key clinical trials for the HeartMate family of devices over the past 20 years are shown in Table 2. Endpoints have evolved over 2 decades due to lengthening durations of support, changes in the technology, and changes in clinical practice. Following the dismal outcome of medically treated shock patients in REMATCH, clinical trials now compare new LVAD technologies in a noninferiority evaluation compared to predicate devices with some trials incorporating the opportunity to evaluate for superiority if noninferiority is achieved. This trial design has not changed since there are no new medical interventions for patients with ambulatory, sub-acute cardiogenic shock who are considered LVAD candidates. Several new medical interventions are now available for American College of Cardiology/American Heart Association (ACC/AHA) Stage C HF patients, such as sacubitril/valsartan, but LVAD candidates were systematically excluded from trials examining new medical interventions. Therefore, new medication discoveries are expected to delay disease progression of ambulatory heart failure to ACC/AHA Stage D refractory NYHA class IV HF symptoms, but once patients progress to this point, no novel intervention would be expected to impact the very high mortality. This is important because long-term MCS is now associated with over 80% 2-year survival, while REMATCH found 92% 2-year mortality.
Table 2. Primary and Secondary Endpoints in Key Clinical Trials for HeartMate Devices
| Device | Year* | Study Type | Indication | Primary Endpoint(s) | Secondary Endpoint(s) |
| HeartMate IP (49) | 1995 | Nonrandomized study with untreated concurrent controls | BTT | Transplantation and survival rates at 1 year | Hemodynamics, NYHA class, adverse events |
| HeartMate VE (14) | 2001 | Nonrandomized study with untreated concurrent controls | BTT | Survival to transplantation and survival after transplantation of treated and control patients | Changes in hemodynamic and biochemical data from baseline to final measurement before transplantation or death |
| HeartMate XVE (15) | 2001 | Randomized, controlled | DT | Death from any cause compared between groups | Incidence of serious adverse events, days of hospitalization, QOL, symptoms of depression, and functional status |
| HeartMate II (19,50) | 2007 | Prospective, noncontrolled | BTT | Transplanted, explanted/recovery, or ongoing support at 6 and 18 months | Overall survival, survival while
receiving support, survival after transplantation, frequency of adverse events, NYHA class, 6MWD, and QOL |
| HeartMate II (20) | 2009 | Randomized controlled (HeartMate XVE vs. II) | DT | Composite endpoint at 2 years, survival free from disabling stroke and reoperation to repair or replace device | Survival, frequency of adverse events, QOL, and functional capacity |
| HeartMate II (37-39) | 2015 | Prospective, observational (LVAD vs. OMM) | DT | Composite endpoint at 12 months and 2 years, survival on original therapy with improvement in 6MWD ≥75 m |
|
| HeartWare HVAD vs. HeartMate II (35) | 2017 | Randomized, controlled, noninferiority | DT | Composite of survival free from disabling stroke with patient alive with originally implanted device, having undergone transplantation, or with device explanted because of recovery | Incidence of major adverse events, overall survival, QOL NYHA class, and 6MWD. |
| HeartMate 3 vs. HeartMate II (2,3,36) | 2017 | Randomized controlled, noninferiority | Either BTT or DT | Composite of survival free of disabling stroke or survival free of reoperation to replace or removal of the device at 6 months and 2 years | Frequency of adverse events; actuarial survival; NYHA class, 6MWD, and QOL
|
*First publication of results.
6MWD = 6-min walk distance; BTT = bridge to transplant; DT = destination therapy; HF = heart failure; LVAD = left ventricular assist device; NYHA = New York Heart Association; OMM = optimal medical management; QOL = quality of life.
Single Endpoints
Single primary endpoints in early bridge-to-transplant trials were typically survival rates to transplantation or to specific time periods. The primary endpoint of the landmark REMATCH trial published in 2001 was all-cause mortality compared between the LVAD and control (medical therapy) arms (15). Secondary endpoints included adverse events, hospitalization time, QOL, and functional status. The BTT studies with the IP-LVAD and VE LVAD had average support durations of only 76 days and 112 days, respectively. But in the HeartMate II DT study, the median duration was 1.7 to 1.8 years, and some of these patients continue to be supported after 10 years (20,32). Survival rates have steadily increased as MCS technology has improved and patient selection has been refined. The 1-year survival rates reported to the INTERMACS registry, which accounts for all United States patients receiving LVAD support, increased from 56% in 2008 to 83% in 2018 (33,34).
Primary Composite Endpoints
Primary endpoints have changed from survival rates at specified timepoints to composites that include patient survival with freedom from 1 or 2 major adverse events. It is no longer sufficient to just be alive — the patient must also be free of major complications and have a reasonable QOL. Composite endpoints were introduced in the HeartMate II DT trial with the primary endpoint of survival free from disabling stroke or reoperation to repair or replace the device. This composite endpoint reflected the past experience with the control device, the HeartMate XVE, which had high rates of mechanical failure and device infection that often resulted in device replacement. In the ENDURANCE trial for the DT indication comparing the HeartWare HVAD with the HeartMate II as the control (35), the primary and secondary endpoints were nearly identical to the HeartMate II versus HeartMate XVE trial.
The primary endpoint in the MOMENTUM 3 trial was survival free of disabling stroke or survival free of reoperation to replace or remove the device (2,3,36). The adaptive design of the MOMENTUM trial had three prespecified analyses: the short-term primary-endpoint assessment in 294 patients at 6 months; a long-term primary-endpoint assessment in 366 patients at 2 years; and a full cohort analysis of the endpoint in 1,028 patients at 2 years. Results showed the HeartMate 3 to be superior to the HeartMate II in reaching this endpoint (Figure 2) (3).
Figure 2. MOMENTUM 3: Primary Endpoint (Kaplan-Meier Estimates in the Intention-to-Treat Population)
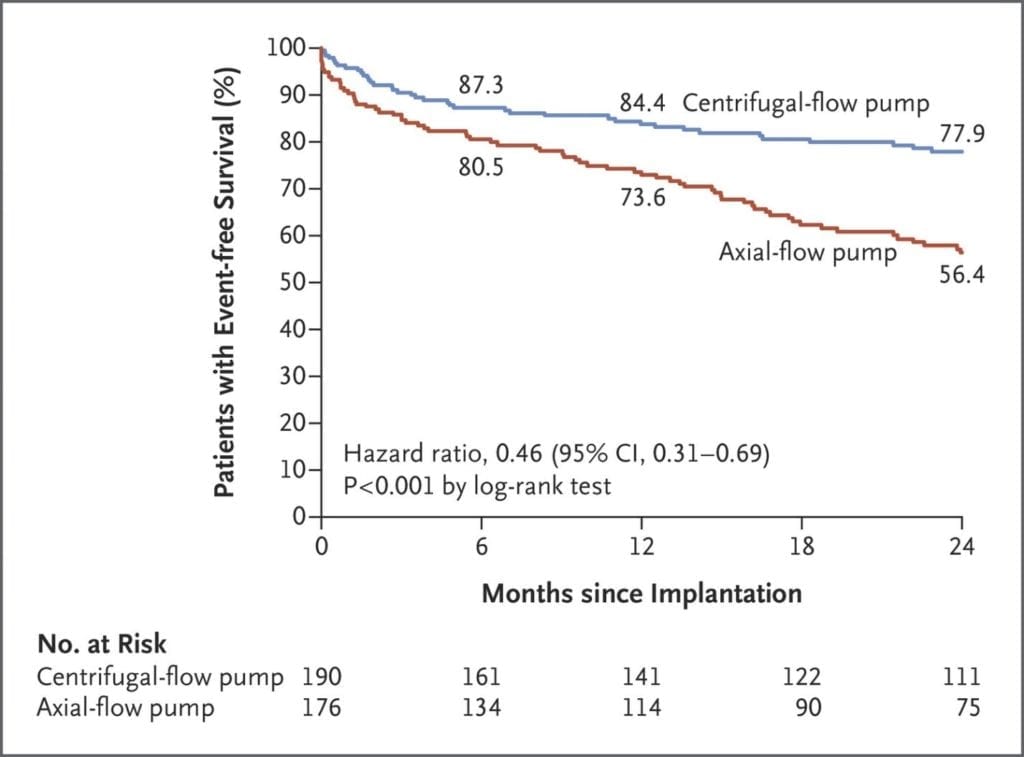
The primary endpoint was a composite of survival at 2 years free from disabling stroke or reoperation to replace or remove a malfunctioning device. The study showed the HeartMate 3 centrifugal-flow pump to be superior to the axial-flow HeartMate II pump. (Reprinted with permission from Mehra MR, et al. N Engl J Med 2018;378:1386-95.)
As survival rates have improved and leveled, it is more difficult to demonstrate improvements or differences between LVAD systems in survival; thus, inclusion of either a functional measure or a QOL metric becomes more relevant. In the post-market ROADMAP (Risk Assessment and Comparative Effectiveness of Left Ventricular Assist Device and Medical Management in Ambulatory Heart Failure Patients) study, less severely ill patients receiving LVADs were compared to medical management by using a composite primary endpoint of survival at 2 years with an increase in 6MWT distance of at least 75 meters (37-39). The ROADMAP study showed that survival on original therapy with improvement in 6MWT distance was better with the LVAD compared with medical therapy at 1 and 2 years (37,39). A similar primary endpoint was used in the NHLBI-sponsored REVIVE-IT (Randomized Evaluation of VAD Intervention before Inotropic Therapy) trial, which was intended to compare the effectiveness of LVAD and medical therapy in non-inotrope-dependent patients through a design randomizing patients to LVAD or medical therapy. Unfortunately, that trial was not completed and evolved into a registry (40,41).
Other Secondary Endpoints and Analyses
Other secondary analyses have been published related to hemocompatibility, QOL, functional capacity, and resource utilization, providing endpoints that should be considered in future prospective trial designs.
Hemocompatibility
In a secondary analysis in the MOMENTUM trial, adverse events attributable to LVAD-related bleeding or thrombosis were classified as hemocompatibility-related adverse events (HRAEs) (42). The HRAEs include bleeding, stroke, other neurological events, and suspected or confirmed pump thrombosis. The results demonstrated a significant increase in freedom from HRAEs for the HeartMate 3 compared to the HeartMate II, and the main driver for this was a reduction in nondisabling stroke and pump thrombosis.
QOL in Composite Endpoints
Secondary endpoints that define a good quality of life outcome versus a poor outcome could include survival combined with QOL measures. In one study using the INTERMACS registry, a poor outcome was defined as either death or an average KCCQ score of <45 during the year after LVAD implantation (43). Hypothesis-generating endpoints were also studied in the ROADMAP trial, including a patient-reported HR-QOL assessment using the EuroQol questionnaire and visual analogue scale (VAS) (37). Success was determined as the percentage of those alive at 2 years with an acceptable QOL, such as VAS ≥60 (44). In the ROADMAP study, LVAD therapy resulted in improvement of patient health status in HF patients with low baseline self-reported HR-QOL, but not in patients with acceptable QOL at the time of LVAD implantation (44). These findings may serve to inform subsequent studies with MCS in less ill patients. Patients with acceptable QOL may opt not to proceed with MCS as mortality in this population may be less than the REMATCH trial.
In another secondary analysis of the MOMENTUM trial, functional capacity and HR-QOL assessments were compared between the HeartMate 3 and HeartMate II devices and an additional analysis evaluated the effects of serious adverse events on a composite endpoint designated as “living well on left ventricular assist system (LVAS)” (45). The endpoint was defined as alive on support with a satisfactory HR-QOL score (KCCQ overall score >50) and NYHA class I/II or 6MWT >300 m. The results showed that 65% of patients were living well on LVAS at 6 months after implantation. Patients “living well on LVAS” were younger with higher preoperative hemoglobin values and better baseline QOL and functional capacity than the other patients. This novel composite is more disease specific and may provide physicians and patients with a better metric when considering LVAD implantation. It is expected that this endpoint will be further assessed, particularly with QOL assessment tools that target the LVAD population.
Cost Effectiveness
The cost effectiveness of LVAD therapy is an important consideration for reimbursement agencies and the overall acceptance of the technology, and thus provides a useful secondary endpoint. As LVAD technology and patient management has improved, the overall cost effectiveness of this therapy has improved considerably. In particular, with the reduction of major adverse events, such as bleeding, infection, and respiratory failure as seen in studies with continuous-flow LVADs, the quality-adjusted life-year (QALY) saved has improved substantially (46,47). In the MOMENTUM trial, the average cumulative cost per patient-year in patients supported by the HeartMate 3 was 50.8% lower than for those with the HeartMate II, ($37,685 vs. $76,599) (48). The key drivers for the costs were the number of hospitalizations and the number of days in the hospital resulting from adverse events. Composite endpoints using incremental cost-effectiveness ratio and cost per QALY with survival can provide useful information about the overall utility of LVAD therapy.
Conclusions
Clinical assessment of LVADs has evolved from simply surviving a terminal illness to surviving without adverse events. Seldom is advanced HF realistically compared to other lethal illnesses, such as cancer, but it is interesting how different standards of clinical trial outcomes are applied to interventions targeting the different illnesses. For some reason, advanced HF with a 92% 2-year death rate is not considered as important as most solid tumor cancers. In fact, outcomes of cancer interventions many times quantify chance of tumor regression with little or no evaluation of quality of extended life.
It is time to recognize that the diagnosis of advanced heart failure is a death sentence, with a realistic chance of 1-year survival of 50%. Mechanical circulatory support is now a viable and very effective means to change the prognosis of inevitable death in patients with a diagnosis of ‘advanced heart failure’. Concerted efforts to decrease the chances of adverse events that decrease quality of extended life, such as stroke or gastrointestinal bleeding, is inherent in the evolution of LVAD clinical trials. Allowing patients to choose between a greater than 90% chance of 2-year mortality or greater than 80% chance of survival must be balanced with nonfatal endpoints that may significantly impair quality of the extended life offered. With this in mind, endpoints of modern LVAD studies have consistently quantified post-operative ill effects to allow true informed consent when considering extended life expectancy. Future technology will continue to address adverse effects in patients with long-term MCS support, which will require focus on endpoints quantifying living free of ill-effects.
References
- Miller LW, Rogers JG. Evolution of Left Ventricular Assist Device Therapy for Advanced Heart Failure: A Review. JAMA Cardiol. 2018;3:650-8.
- Mehra MR, Naka Y, Uriel N, et al. A Fully Magnetically Levitated Circulatory Pump for Advanced Heart Failure. N Engl J Med. 2017;376:440-50.
- Mehra MR, Goldstein DJ, Uriel N, et al. Two-Year Outcomes with a Magnetically Levitated Cardiac Pump in Heart Failure. N Engl J Med. 2018;378:1386-95.
- Pierce WS, Donachy JH, Landis DL et al. Prolonged mechanical support of the left ventricle. Circulation. 1978;58:I133-46.
- Norman J BM, Cooley D, Klima T, Kahan B. Total support of the circulation of a patient with post-cardiotomy stone-heart syndrome by a partial artificial heart (AL-VAD) for 5 days followed by heart and kidney transplantation. Lancet. 1978;1:1125-27.
- Norman J, Dasco CC, Reul GJ, et al. Partial artificial heart (ALVAD) use with subsequent cardiac and renal allografting in a patient with stone heart syndrome. Artif Organs. 1978;2:413-20.
- Hill JD, Farrar DJ, Hershon JJ, et al. Bridge to cardiac transplantation: successful use of prosthetic biventricular support in a patient awaiting a donor heart. ASAIO Trans. 1986;32:233-7.
- Farrar DJ, Holman WR, McBride LR, et al. Long-term follow-up of Thoratec ventricular assist device bridge-to-recovery patients successfully removed from support after recovery of ventricular function. J Heart Lung Transplant. 2002;21:516-21.
- Farrar DJ, Hill JD, Gray LA Jr, et al. Heterotopic prosthetic ventricles as a bridge to cardiac transplantation. A multicenter study in 29 patients. N Engl J Med. 1988;318:333-40.
- Farrar DJ, Hill JD, Pennington DG, et al. Preoperative and postoperative comparison of patients with univentricular and biventricular support with the thoratec ventricular assist device as a bridge to cardiac transplantation. J Thorac Cardiovasc Surg. 1997;113:202-9.
- Frazier OH. Chronic left ventricular support with a vented electric assist device. Ann Thorac Surg. 1993;55:273-5.
- Frazier OH. First use of an untethered, vented electric left ventricular assist device for long-term support. Circulation. 1994;89:2908-14.
- Myers TJ, Dasse KA, Macris MP, Poirier VL, Cloy MJ, Frazier OH. Use of a left ventricular assist device in an outpatient setting. ASAIO J. 1994;40:M471-5.
- Frazier OH, Rose EA, Oz MC, et al. Multicenter clinical evaluation of the HeartMate vented electric left ventricular assist system in patients awaiting heart transplantation. J Thorac Cardiovasc Surg. 2001;122:1186-95.
- Rose EA, Gelijns AC, Moskowitz AJ, et al. Long-term mechanical left ventricular assistance for end-stage heart failure. N Engl J Med. 2001;345:1435-43.
- Griffith BP, Kormos RL, Borovetz HS, et al. HeartMate II left ventricular assist system: from concept to first clinical use. Ann Thorac Surg. 2001;71:S116-20; discussion S114-6.
- Frazier OH, Myers TJ, Westaby S, Gregoric ID. Use of the Jarvik 2000 left ventricular assist system as a bridge to heart transplantation or as destination therapy for patients with chronic heart failure. Ann Surg. 2003;237:631-6; discussion 636-7.
- Song X, Throckmorton AL, Untaroiu A, et al. Axial flow blood pumps. ASAIO J. 2003;49:355-64.
- Miller LW, Pagani FD, Russell SD, et al. Use of a continuous-flow device in patients awaiting heart transplantation. N Engl J Med. 2007;357:885-96.
- Slaughter MS, Rogers JG, Milano CA, et al. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med. 2009;361:2241-51.
- Patel SM, Throckmorton AL, Untaroiu A, Allaire PE, Wood HG, Olsen DB. The status of failure and reliability testing of artificial blood pumps. ASAIO J. 2005;51:440-51.
- John R, Kamdar F, Liao K, Colvin-Adams M, Boyle A, Joyce L. Improved survival and decreasing incidence of adverse events with the HeartMate II left ventricular assist device as bridge-to-transplant therapy. Ann Thorac Surg. 2008;86:1227-34; discussion 1234-5.
- Radovancevic B, Vrtovec B, de Kort E, Radovancevic R, Gregoric ID, Frazier OH. End-organ function in patients on long-term circulatory support with continuous- or pulsatile-flow assist devices. J Heart Lung Transplant. 2007;26:815-8.
- Kamdar F, Boyle A, Liao K, Colvin-Adams M, Joyce L, John R. Effects of centrifugal, axial, and pulsatile left ventricular assist device support on end-organ function in heart failure patients. J Heart Lung Transplant. 2009;28:352-9.
- Slaughter MS. Long-term continuous flow left ventricular assist device support and end-organ function: prospects for destination therapy. J Card Surg. 2010;25:490-4.
- McIlvennan CK, Magid KH, Ambardekar AV, Thompson JS, Matlock DD, Allen LA. Clinical outcomes after continuous-flow left ventricular assist device: a systematic review. Circ Heart Fail. 2014;7:1003-13.
- Kirklin JK, Pagani FD, Kormos RL, et al. Eighth annual INTERMACS report: Special focus on framing the impact of adverse events. J Heart Lung Transplant. 2017;36:1080-1086.
- Rogers JG, Aaronson KD, Boyle AJ, et al. Continuous flow left ventricular assist device improves functional capacity and quality of life of advanced heart failure patients. J Am Coll Cardiol. 2010;55:1826-34.
- Glick HA, Polsky D, Willke RJ, Schulman KA. A comparison of preference assessment instruments used in a clinical trial: responses to the visual analog scale from the EuroQol EQ-5D and the Health Utilities Index. Med Decis Making. 1999;19:265-75.
- Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol. 2000;35:1245-55.
- Middel B, Bouma J, de Jongste M, et al. Psychometric properties of the Minnesota Living with Heart Failure Questionnaire (MLHF-Q). Clin Rehabil 2001;15:489-500.
- Park SJ, Milano CA, Tatooles AJ, et al. Outcomes in advanced heart failure patients with left ventricular assist devices for destination therapy. Circ Heart Fail. 2012;5:241-8.
- Kirklin JK, Naftel DC, Stevenson LW, et al. INTERMACS database for durable devices for circulatory support: first annual report. J Heart Lung Transplant. 2008;27:1065-72.
- Kormos RL, Cowger J, Pagani FD, et al. The Society of Thoracic Surgeons Intermacs database annual report: Evolving indications, outcomes, and scientific partnerships. J Heart Lung Transplant. 2019;38:114-126.
- Rogers JG, Pagani FD, Tatooles AJ, et al. Intrapericardial Left Ventricular Assist Device for Advanced Heart Failure. N Engl J Med. 2017;376:451-460.
- Heatley G, Sood P, Goldstein D, et al. Clinical trial design and rationale of the Multicenter Study of MagLev Technology in Patients Undergoing Mechanical Circulatory Support Therapy With HeartMate 3 (MOMENTUM 3) investigational device exemption clinical study protocol. J Heart Lung Transplant. 2016;35:528-36.
- Estep JD, Starling RC, Horstmanshof DA, et al. Risk Assessment and Comparative Effectiveness of Left Ventricular Assist Device and Medical Management in Ambulatory Heart Failure Patients: Results From the ROADMAP Study. J Am Coll Cardiol. 2015;66:1747-61.
- Rogers JG, Boyle AJ, O’Connell JB, et al. Risk assessment and comparative effectiveness of left ventricular assist device and medical management in ambulatory heart failure patients: design and rationale of the ROADMAP clinical trial. Am Heart J. 2015;169:205-210.e20.
- Starling RC, Estep JD, Horstmanshof DA, et al. Risk Assessment and Comparative Effectiveness of Left Ventricular Assist Device and Medical Management in Ambulatory Heart Failure Patients: The ROADMAP Study 2-Year Results. JACC Heart Fail. 2017;5:518-27.
- Pagani FD, Aaronson KD, Kormos R, et al. The NHLBI REVIVE-IT study: Understanding its discontinuation in the context of current left ventricular assist device therapy. J Heart Lung Transplant. 2016;35:1277-83.
- Baldwin JT, Mann DL. NHLBI’s program for VAD therapy for moderately advanced heart failure: the REVIVE-IT pilot trial. J Card Fail. 2010;16:855-8.
- Uriel N, Colombo PC, Cleveland JC, et al. Hemocompatibility-Related Outcomes in the MOMENTUM 3 Trial at 6 Months: A Randomized Controlled Study of a Fully Magnetically Levitated Pump in Advanced Heart Failure. Circulation. 2017;135:2003-12.
- Arnold SV, Jones PG, Allen LA, et al. Frequency of Poor Outcome (Death or Poor Quality of Life) After Left Ventricular Assist Device for Destination Therapy: Results From the INTERMACS Registry. Circ Heart Fail. 2016;9.
- Stehlik J, Estep JD, Selzman CH, et al. Patient-Reported Health-Related Quality of Life Is a Predictor of Outcomes in Ambulatory Heart Failure Patients Treated With Left Ventricular Assist Device Compared With Medical Management: Results From the ROADMAP Study (Risk Assessment and Comparative Effectiveness of Left Ventricular Assist Device and Medical Management). Circ Heart Fail. 2017;10.
- Cowger JA, Naka Y, Aaronson KD, et al. Quality of life and functional capacity outcomes in the MOMENTUM 3 trial at 6 months: A call for new metrics for left ventricular assist device patients. J Heart Lung Transplant. 2018;37:15-24.
- Slaughter MS, Bostic R, Tong K, Russo M, Rogers JG. Temporal changes in hospital costs for left ventricular assist device implantation. J Card Surg. 2011;26:535-41.
- Rogers JG, Bostic RR, Tong KB, Adamson R, Russo M, Slaughter MS. Cost-effectiveness analysis of continuous-flow left ventricular assist devices as destination therapy. Circ Heart Fail. 2012;5:10-6.
- Mehra MR, Salerno C, Cleveland JC, et al. Healthcare Resource Use and Cost Implications in the MOMENTUM 3 Long-Term Outcome Study. Circulation. 2018;138:1923-34.
- Frazier OH, Rose EA, McCarthy P et al. Improved mortality and rehabilitation of transplant candidates treated with a long-term implantable left ventricular assist system. Ann Surg. 1995;222:327-36; discussion 336-8.
- Pagani FD, Miller LW, Russell SD, et al. Extended mechanical circulatory support with a continuous-flow rotary left ventricular assist device. J Am Coll Cardiol. 2009;54:312-21.
