Translational Pathway for Transcatheter Mitral and Tricuspid Valve Development
Clinical Evaluations – Endpoints
Authors
Blasé Carabello, MD
Anthony DeMaria, MD
David Holmes, MD
Spencer King III, MD
John Laschinger, MD
Patrick Verta, MS, DVM, MD
Blasé Carabello, MD
Mitral Valve Devices
General Principles: Safety and Efficacy
In considering the approval and success of any device, safety and effectiveness will be considered paramount and each will be evaluated in the context of a comparator based on the current standard of care. For the purposes of device-based therapies, primary and secondary mitral regurgitation (MR) should be viewed as two nearly separate diseases. They have different pathologies, different outcomes, and different therapeutic needs. Thus, in establishing endpoints for the judgement and success of a proposed therapy it is logical that these will be different for the two diseases.
It is also important that evaluations be conducted by a multispecialty heart team, with experienced members including a heart failure cardiologist, an echocardiographer, an interventionalist, and mitral valve (MV) surgeon, to ensure all aspects of the evaluation are viewed through a critical and relevant medical lens.
Primary MR: Treatment Can Be “Curative”
There are no randomized trials comparing MV repair to MV replacement in primary MR, and it is highly unlikely that there would ever be equipoise enabling such a trial to be conducted. Mitral valve repair is the accepted treatment of choice for reparable valves, as it produces <1% mortality in experienced hands, has ~1%/year failure rate, and may return lifespan to normal. However, important caveats exist. Outcomes vary with surgical experience, which is why current guidelines recommend that patients be referred to a “center of excellence” for surgery (1). Nonetheless, it should be anticipated that a comparator device would have to have similar outcomes even if it were less invasive. Obviously, surgical risk varies with the condition of the patient being treated, and there is surely a crossover point where patients at high risk for surgery would be better treated with a less invasive device. This paradigm has worked well for the evaluation of transcatheter aortic valve replacement (TAVR) wherein the bar for comparison was progressively lowered from comparing TAVR first to inoperable patients (2), then to high-risk surgical patients (3), then to intermediate-risk patients (4), and recently to low-risk patients (5,6).
Thus, for patients early in the course of primary MR, prior to left ventricular (LV) dysfunction and with minimal symptoms, a well-performed surgical repair that addresses all three levels of the valve complex and results in correction of MR is the standard of care. For patients later in the course of primary MR or for those with severe comorbidities, less invasive treatments that fail to address the entire valve complex but result in significant reduction of MR and improvements in symptoms or quality of life (QOL) may play a role. Additional considerations with regard to choice of the patient population include operative risk status and timing in disease course, including the severity of resulting or underlying ventricular remodeling and dysfunction and the severity of symptoms.
Assessing Safety
Since test and control therapies will have different safety concerns, it is important that the safety endpoints used to evaluate the test and control therapy be balanced. The variations in both procedural and device safety risks posed by various combinations of drug, surgical or transcatheter therapy and their timing must be accounted for in the design of any study. When used as a composite, important procedural or device related adverse events must be balanced to avoid designs unfairly favor one therapy over another where possible. Additionally, all adverse events related to procedural safety or device safety specific to the assigned treatment must also be carefully evaluated and adjudicated appropriately. Important device safety concerns include those related to access (e.g., vascular, apical, septal, open surgical), anchor points (e.g., tissue disruption, device misplacement, embolism), durability (e.g., malfunction, fracture, thrombosis), damage or interaction with surrounding tissues (e.g., mitral apparatus, coronary compression), infection, paravalvular leak, hemolysis, and unplanned additional devices, and need for emergent unplanned intervention or surgery.
Major safety objective measures:
- Procedural mortality
- Stroke
- Hemorrhage
- Renal injury
- Myocardial infarction
Performance:
- MR reduction
- Durability
- Stability
Assessment of Effectiveness
Determining the proper endpoint for device effectiveness depends on the etiology of MR, the hemodynamic goals of the device (correction vs. reduction of MR), the available comparative therapies, the timing of treatment in the course of the disease and the incidence and effects of residual MR or MR recurrence. Typical endpoints will be reflective of improvements in survival. Reductions in heart failure (HF) events may need to be supplemented by effectiveness endpoints designed to assess the occurrence and durability of symptom palliation or measurable improvements in function or quality of life.
Major effectiveness objective measures:
- Reduced or equivalent mortality
- Reduced hospitalization for heart failure
- Improvement in symptoms
- Improvement in function
- Improved QOL
- Reversed remodeling
- Improved biomarkers
Endpoints for Operable Patients With Primary MR
For operable patients with primary MR, mitral valve repair (MVRe) for myxomatous valves is the clear gold standard for any comparative device. It is assumed that such a device would be of utility only if it offered a less invasive alternative to MVRe. The device would have to be deployed with similar mortality; cause a substantial, durable reduction in MR; and produce equal or better long-term mortality and QOL, with equivalent improvements in symptoms and function if present pre-procedurally. Imaging indexes of heart function and biomarkers should be considered for inclusion as secondary endpoints, but there is not yet sufficient evidence to permit their use as primary or independent indicators of effectiveness for regulatory purposes.
For patients who require mitral valve replacement, a new device should have equal or reduced mortality and hold the other tenets noted above.
Endpoints for Inoperable Patients for Primary MR
For inoperable patients the current standard of care is medical therapy, although alternative device-based therapy is available. Current guidelines recommend that for severely symptomatic patients with severe chronic MR, “Transcatheter MV repair may be considered in patients who have a reasonable life expectancy but a prohibitive surgical risk because of severe comorbidities” (1). A new device for use in this population might not be expected to alter life expectancy, but it should substantially and durably reduce MR without procedural morbidity/mortality or long-term device risks that are out of proportion to the probable benefits. In these patients, demonstrated benefits that might substantiate a new alternative or standard for care would include improvements in symptoms, function or QOL with or without associated reductions in the need for HF-related hospitalizations while improving QOL and hopefully reducing hospitalizations for heart failure (HHF).
Efficacy Endpoints for Secondary MR Repair Device Trials
Secondary MR is labeled as such because it is secondary to disease of the left ventricle (LV) either from myocardial infarction or cardiomyopathy. Comparisons of surgery to medical therapy were nearly unanimous in failing to find a mortality benefit to surgery, and until recently there has been no convincing evidence that treating secondary MR improved mortality. Thus, recent studies in the field of secondary MR have used a relatively new statistical methodology, the Finklestein-Schoenfeld (FS) analysis, or a variation of it, the win ratio (7), to allow meaningful and important comparisons of weighted composite endpoints among treatment groups. The FS methodology addresses problems arising from the mixing of components of different nature and severity in a single composite endpoint. By attributing a higher weight, or ranking, to the most clinically important components, these methods provide a coherent framework on which complex composite endpoints can be analyzed, thus addressing the issues associated with the disparity in severity and clinical importance of the various components. It then allows separation and weighting of outcomes according to those most important to the patient. If, for example, a device had no effect on mortality but improved QOL, it would be considered a “win” for quality and a draw for mortality. This method allows for a reduced sample size and more efficacious studies.
The shift from a traditional trial design to a contemporary methodology in recent secondary MR repair studies has several important consequences. The FS methodology allowed the migration of patient-reported outcome secondary endpoints to the status of primary endpoints. Along with mortality and HHF, the Kansas City Cardiomyopathy Questionnaire, a QOL assessment designed for HF patients, and the 6-min walk test (6MWT), a submaximal level of exercise capacity, are now components of the primary composite endpoints of two transcatheter mitral valve repair studies, ACTIVE (NCT03016975), evaluating the CardioBand system, and CARILLON (NCT03142152), assessing the Carillon mitral contour system. Those endpoints, considered “softer“ than traditional secondary endpoints, are now components of the primary endpoint while still considered as secondary endpoints on their own. This is important and reflects the most current thinking in clinical trials, where what matters most to patients is now prioritized along with what matters most to clinicians, regulators, or payers.
Sample size depends on two main factors: numbers of events and the expected difference between treatment arms, or effect size. In the FS methodology, with components that are not “events” per se such as QOL scores or continuous variables (like the 6MWT) where, in theory, there should be a record for every patient alive at the end of the study period, the sample size is reduced. One important consideration with these new endpoints is the possibility that the study could be positive (i.e., a p value <0.05) on the basis of QOL and 6MWT, with no detectable effect on mortality and HHF. Because these newer studies are not powered to show a statistically significant reduction in mortality and HHF, even if a difference is observed at the end of the study for any of these endpoints, there is a chance that the difference would be nominal and non-statistically significant. Some of the components could, by chance alone, go in the opposite direction of the overall result; consistency in the predicted and observed directions of outcomes is a key factor in study design and analysis.
Another challenge is the interpretation of studies using these ranked composite endpoints. Physicians are used to reporting hazard ratios for mortality and/or HHF and improvements from baseline for exercise capacity and QOL. A derivative of the FS methodology, the win ratio provides more clarity. The win ratio itself can be interpreted as the increased risk for a patient taken at random from the control group compared to a patient taken at random from the treatment group of reaching an event first. The win ratio can be computed for the whole composite endpoint as well as for each individual component. The question remains, however, as to how regulators, payers, clinicians, patients and guideline writing committee will interpret these results.
In summary, the ranked composite endpoint now used in two randomized clinical trials (RCTs) of transcatheter MVr devices has several advantages over more traditional endpoints as it: includes multiple components of various severity; ranks the relative severity (weight) of components; allows for a smaller sample size; and allows inclusion of patient-reported outcomes as part of the primary endpoint. It also carries significant challenges: how should the primary endpoint result be interpreted? What components drive a positive outcome? How should discordance among components be interpreted? And how do other stakeholders view these endpoints?
The Effect of “COAPT” on These Concepts
One RCT of percutaneous therapy for secondary MR using the MitraClip™, the Mitra-FR study, found no mortality or HHF benefit to that therapy over optimal medical management (8). However, another well-performed trial, the COAPT trial, found that this therapy not only reduced repeat HHF but also reduced mortality in patients with secondary MR (9). The safety profile was also excellent. Although the patients in COAPT had severe LV dysfunction, their ventricles had less severe LV dilatation and more severe MR than did the patients in Mitra-FR. Importantly, patients entered into COAPT underwent centrally monitored guideline-directed medical therapy (GDMT) that typically utilized beta-blockers, diuretics, and blockade of the renin-angiotensin-aldosterone system, and cardiac resynchronization therapy when indicated. Many patients proposed for enrollment were rejected until it was proven that MR remained severe after therapy had been maximized. Thus, it is likely that the success of COAPT was also based in part upon the potentialization of two therapies, GDMT in concert with the MR reduction caused by the device.
Therefore, it seems unlikely the device would be successful as a single therapy. COAPT plus GDMT may set a new standard for the treatment of secondary MR, especially if it is confirmed in another trial. Does this mean that all new devices will be required to use MitraClip plus GDMT as the comparator? There is no longer equipoise allowing randomization against GDMT alone in a COAPT-like population. The TAVR trials may provide an important example. Once the PARTNER I B study proved TAVR superior to medical therapy in extreme risk patients (20% mortality benefit) (2), CoreValve redesigned their trial, using a prespecified mortality performance goal instead of randomization in their first trial. Thus, subsequent device trials could be similarly designed if COAPT results can be confirmed. Interestingly, the COAPT and Mitra-FR trials might have defined a high-risk group that did not benefit from the MitraClip. From these data inclusion/exclusion criteria for a trial could be tailored to allow patient randomization into other device trials where equipoise would still exist. It is clear that any new device must be applied to well-selected patients, and that trial designs must account for devices applied at different points in the disease process with different therapeutic goals (i.e., MR correction vs. MR reduction). In order to select appropriate patients for application of any device, application of the heart team concept is crucial. In this construct, a multidisciplinary group of clinicians reviews all data available and discusses each patient, deciding upon the appropriate therapy for that patient based upon his/her clinical status. The team would need to be constructed to assure that stringent definitions for quantification of MR and underlying ventricular function were met at baseline and at important follow-up intervals, that requirements for concomitant medical therapy both pre- and post-procedure were standardized by type and compliance, and that the choice of procedure and its performance were optimized for the patients being studied.
It should be noted that COAPT encountered the issue of competing risk with mortality and resulting in loss of power. This issue was mitigated, while the sponsor was still blinded to the study results and the study was still enrolling, by the modification of the primary endpoint analysis to a joint-frailty model. This accounted for the fact that cardiovascular mortality was informative and not merely a censoring event.
Endpoints for Patients With Secondary MR
The standard of care for secondary MR has been GDMT. It would be expected that the device would provide enough reduction in MR to be effective. Although longevity is reduced in secondary MR patients, the device would have to be durable enough to last for the patients’ expected life span. Time will tell whether COAPT becomes the new standard of care for subsets of patients with severe secondary MR.
Patient Perspective
While the above endpoints are of key importance to cardiologists, regulators, and industry, ultimate success of any device will have to include the individual patient’s experience regarding implantation and the effects of the device on his/her QOL.
The gradation of importance for primary and secondary MR is shown in Figure 1. In primary MR (left) a long asymptomatic life post implantation is a reasonable goal whereas for secondary MR (right) improved quality of life is key.
Figure 1. Effectiveness Outcomes Important to Patients
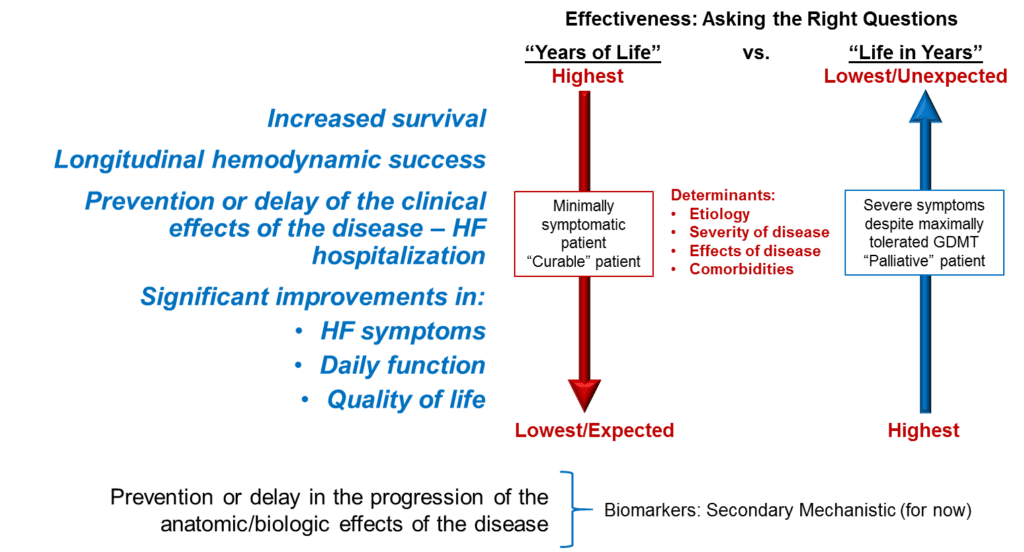
Device or treatment efficacy may mean different things to different patients, particularly when disease severity is considered. For example, for minimally symptomatic patients who are not experiencing concerns regarding daily functions, increased survival will likely be a more critical outcome whereas patients with more severe disease are likely to be focused on the quality, rather than length, of life before them. Dz = disease; GDMT = guideline-directed medical therapy; HF = heart failure.
Tricuspid Valve Devices
General Principles
Our understanding of the importance of tricuspid regurgitation (TR) is decades behind that of MR, making specific endpoints more difficult to target. Nonetheless, TR (like MR) is categorized as either primary and secondary with most cases of TR secondary to right ventricular volume or pressure overload often created by left-sided failure. A frequent scenario is the emergence of secondary TR following successful correction of left-sided lesions wherein the TR was felt to be mild (and left untreated) at the time of surgery. Patients are then left with right-sided failure due to TR and require mechanical intervention yet repeat surgery is at high risk, making a percutaneous approach attractive.
Endpoints
— Safe delivery: Because risk assessment for isolated surgical tricuspid valve repair or replacement is lacking, it is difficult to have a safety benchmark for comparison with non-surgical devices. Thus, risk models must be developed to assess risk performance of devices as they are tested.
— Reduction in TR: In contrast to MR, the amount of TR reduction needed to cause a clinical benefit is unknown. Further, quantification of TR is less succinct and less commonly practiced than is quantification of MR. While it is generally agreed that MR device success should be defined as reduction to moderate MR or less, it may be that reduction in TR from torrential to severe may be of benefit. Therefore, at present, TR device success will be judged primarily by clinical improvement.
— Clinical improvement: Following device implantation there should be both subjective and objective evidence of improvement. These include self-reporting of symptom status, QOL questionnaires, rehospitalizations, and 6MWTs.
Assessment of TR severity, accepted triggers for TR intervention, and definitions of success for those interventions are evolving and not yet standard. Likewise, the amount of TR reduction necessary to cause clinical improvement is not defined. All of these issues must be addressed in order to establish endpoints for successful and effective TR device implantation. Until then, success will be defined as device-related subjective and objective clinical improvement.
References
- Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2017;70:252-89.
- Leon MB, Smith CR, Mack M, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597-1607.
- Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364:2187-2198.
- Leon MB, Smith CR, Mack MJ, et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2016;374:1609-1620.
- Waksman R, Rogers T, Torguson R, et al. Transcatheter Aortic Valve Replacement in Low-Risk Patients With Symptomatic Severe Aortic Stenosis. J Am Coll Cardiol. 2018;72:2095-105.
- Mack MJ, Leon MB, Thourani VH, et al. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N Engl J Med. 2019 Mar 17. doi: 10.1056/NEJMoa1814052. [Epub ahead of print]
- Pocock SJ, Ariti CA, Collier TJ, Wang D. The win ratio: a new approach to the analysis of composite endpoints in clinical trials based on clinical priorities. Eur Heart J. 2012;33:176-82.
- Obadia JF, Messika-Zeitoun D, Leurent G, et al. Percutaneous Repair or Medical Treatment for Secondary Mitral Regurgitation. N Engl J Med. 2018;379:2297-2306.
- Stone GW, Lindenfeld J, Abraham WT, et al. Transcatheter Mitral-Valve Repair in Patients with Heart Failure. N Engl J Med. 2018;379:2307-18.
