Translational Pathway for Transcatheter Mitral and Tricuspid Valve Development
Preclinical Evaluation of Percutaneous Mitral/Tricuspid Valve Repair/Replacement
Author
Patrick Verta, MS, DVM, MD
Introduction
The term “preclinical testing” may not cover very well the wide range of activities that are required to develop and prove a certain device’s safety, validity, and verification for specific clinical needs. The process is more than a series of tests. Obviously, none of the preclinical tests may present the variety of human body responses to the device. We need to accept that this phase cannot eliminate the entire risk and fully predict human responses to the technology but plays an important role in developing and demonstrating the device is ready for clinical assessments.
Quality Management System
Any new technology addressing the mitral and tricuspid valves needs to start with an adaptation of a quality management system (QMS) if it is not already established. ISO 13485:2016, which can be found with other standards at the International Organization for Standardization website, offers wide-ranging guidelines covering topics as broad as documentation practices and management responsibilities through product realization and measurement/analysis requirements. These guidelines must also be used by suppliers or other external parties providing products or services. Design and development activities such as planning and understanding inputs and the processes that relate inputs to outputs as well as design verification and validation are all key elements of a QMS.
Process monitoring and measurement is critical to provide consistent product and also to identify bounds on what constitutes acceptable product. These limits must be identified and controlled through manufacturing process validation(s) and subsequent monitoring. A corrective and preventative action (CAPA) process is then implemented to deal with situations outside of process limits.
Design verification testing and validation activities such as preclinical studies are targeted at rigorous evaluation of product representative of an acceptable range of process results such as dimensional requirements. One example of this rigorous evaluation is high cycle fatigue characterization. This design verification test is conducted on product representative of the highest stressed device based on worst-case dimensions and forces resulting from anatomical interaction exaggerated by hypertensive blood pressures and multiplied by an appropriate safety factor. Such considerations are basic requirements of a QMS and ISO 5840.
Design Requirements
One of the first considerations in the development of a mitral or tricuspid valve technology is to understand the design requirements based on the use conditions (Figure 1). This includes a system-level risk assessment that should be initiated early in the design development phase. Design requirements for mitral and tricuspid technologies can be simplified into three fundamental categories: deliverability; structural integrity; and functional performance. Design verification testing and preclinical (in vivo) testing are both conducted to demonstrate that the design outputs, that is, the list of specification blueprints describing how each device is manufactured, meet these design inputs providing some measure of safety for further clinical evaluation.
Figure 1. Design Requirements
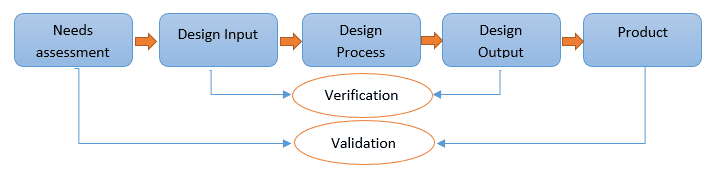
Development of mitral or tricuspid valve technology begins with an assessment of needs and risk to guide the overall design and verification process.
Selecting the right tests based on the design and use conditions is critical to ensure that the testing performed addresses all of the pertinent questions to mitigate risk. Three basic steps can help develop an appropriate test strategy:
- Asking the right questions.
- Developing the right test methods, inspection, or analysis. The tests may include those to assess the physical, chemical, biological, and mechanical properties of deliverables.
- Verifying test methods appropriately challenge the design.
For each of these requirements, individual test reports are provided for each bench and laboratory test, computer modeling analysis (e.g., finite element analysis), and in vivo animal study. Each test report should include the purpose, test method, sample selection, results, discussion of the acceptability of the results, and when appropriate, justification and clinical applicability of the acceptance criteria.
Deliverability
Current ISO guidelines for transcatheter cardiovascular therapies are mainly defined based on the transcatheter aortic valve replacement experience and requirements. For all mitral or tricuspid technologies, these guidelines need to be updated.
Design Inputs
Considering the wide range of pathophysiological conditions that affect mitral and tricuspid valves, it is important to define the intended use, that is which specific condition and population, for which the technology is designed. Based on this definition, the list of adverse events and intended claims will be different.
- Operational specifications: These include the values of operation, intended device delivery approach/process, expected device lifetime, shelf life, and shipping/storage limits. Additionally, a wide range of anatomical variables including but not limited to the annulus size, calcification, and presence of tethering, cleft, prolapse, flail segments and all other anatomical and hemodynamic factors that affect the interaction with the technology must be well defined.
- Performance specifications: Although ISO 5840-3 provides detailed guidance regarding performance specifications of the new technologies, requirements in the mitral and tricuspid technologies could be more complex, including:
- Delivery system
- Safe access with minimal anatomical interaction, delivery, placement and deployment of the intended implant(s) at intended location(s) and planned orientation
- Safe withdrawal of the delivery system
- Minimal anatomical damage to interatrial septum for a transseptal approach to mitral valve or to the ventricle during transapical approach
- Visibility over practical and relevant imaging modalities utilized during the procedure
- Minimal hemolysis,
- Minimal thrombogenicity,
- Minimal bleeding
- Minimal damage to access vessels
- Minimal interaction with other implanted devices
- Minimal air emboli
- Biocompatibility
- Stability
- Flexibility
- Torquability
- Pushability
- Trackability
- Implant: the term of the implant in the mitral and tricuspid side is not limited to the valve and its substitutes, but it must cover any biological or artificial part that intentionally delivers inside the body to cure or improve a target pathology. ISO 14630:2012, Clause 5 defines basic design attribute requirements. The minimal intended performance factors for transcatheter mitral and tricuspid therapy need to cover include:
- Loading into the delivery system: It is important to be able to verify safe and reliable loading of the device into the delivery system. The manufacturer shall demonstrate that the implantable device can be reliably attached to the delivery system in accordance with the instructions for use (IFU) and satisfy attachment performance requirements, such as:
-
- Attachment strength between the device and the delivery system
- No damage to the device or the delivery system
- Crimped diameter
- Crimped shape (uniform or nonuniform)
- Proper orientation of the device into the delivery system
- Dislodgement requirements and force
- Device sterility
- Device rinsing
- Delivery system flushing (de-airing)
- Component dimensional compatibility with ancillary devices
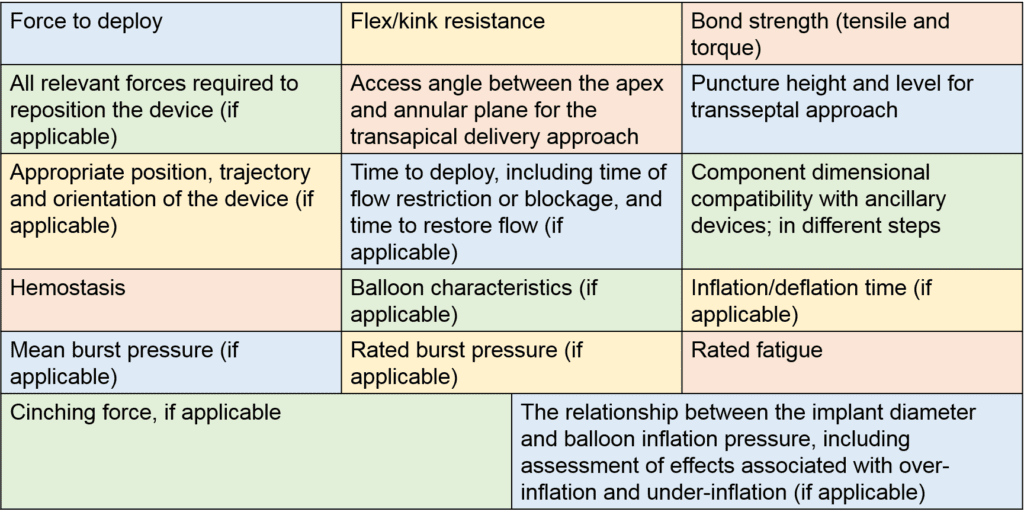
- Deployment: It is important to demonstrate that a delivery system is sufficient to permit safe, repeatable, and reliable delivery of the implant to the intended implant site, release of the device from the delivery system, and safe removal of the delivery system from the patient in accordance with the IFU. The manufacturer shall define all specific performance parameters to be evaluated to verify safe and reliable deployment of the device within the intended implant site (examples listed in Table 1). Other issue related to deployment include:
- Repositioning and retrieval
- Entanglement with chords and other structures
- Stable and fix positioning, retention force to prevent unwanted dislodgement, detachment, partial release, or embolization of implant
- Structural and functional integrity during the expected lifetime of the device
- No or acceptable negative interaction with other anatomical or hemodynamic components, induction of complete heart block, or initiation of the inflammatory response, etc.
- Induction of hemolysis
- Thrombogenicity
- Visibility of device in fluoroscopy, echocardiography, and other relevant imaging modalities
- Compatibility with magnetic resonance imaging
- Interaction with other previously implanted devices
- Biocompatibility
-
- Loading into the delivery system: It is important to be able to verify safe and reliable loading of the device into the delivery system. The manufacturer shall demonstrate that the implantable device can be reliably attached to the delivery system in accordance with the instructions for use (IFU) and satisfy attachment performance requirements, such as:
- Procedural steps: These are as important as a delivery system or implant and need to include:
- Preprocedural activities/requirements including but not limited to:
- Anatomical and hemodynamic assessment
- Required preprocedural lab tests and their acceptable ranges
- Patient preparation, required medication, and anesthetic guide (if applicable)
- Table and system setup over the patient
- Minimum required facilities
- Intraprocedural steps including stepwise main operator and imaging guide
- Postprocedural activities include required medications, hemostasis, and important postprocedural care activities
- Preprocedural activities/requirements including but not limited to:
- Packaging and labeling: It is important to provide enough information about how long a package can maintain a product in a good shape on the shelf. For example, what may happen under a different situation such as freezing or excessive heat? Or which situations should be avoided and which should be monitored? Additionally, in order to make sure users were provided with enough information about the product and how to use it, labels, IFU, and training materials play an important role. Inner and outer labels need to provide the information listed in ISO 5840-3:2013-Annex D including:
- Unit-container label: Each unit container shall be marked with the following information:
- Name or trade name
- Model number
- Serial/lot number
- Size and device type (if applicable)
- The word “sterile” if applicable and the method of sterilization
- For sterile devices, the use by date or the expiration date
- Statement regarding single use only (if applicable)
- Reference to see IFU for user information
- Outer-container label: In addition to applicable storage instructions, each outer container shall be marked with:
- Phrase(s) and/or symbol(s) for:
- Name or trade name of the device;
- Name, address, and phone number of manufacturer and/or distributor and other methods of contacting the manufacturer (e.g., fax number, email address)
- When necessary, name and address of the importer established within the importing country or an authorized representative of the manufacturer established within the importing country
- Model number
- Serial/lot number
- Size and device type (if applicable)
- Net contents
- The word “sterile” and method of sterilization (if applicable)
- h) For sterile devices, the use by date or the expiration date
- Statement regarding single use only (if applicable)
- Devices intended for clinical investigations shall bear identification that the device is intended for investigational use only
- Any special storage or handling conditions as indicated in the device specification
- Warning against use of the device if the unit container has been opened or damaged
- Reference to see IFU for user information
- Phrase(s) and/or symbol(s) for:
- Instructions for use: A good description is contained in ISO 5840-3:2013-Annex D
- Unit-container label: Each unit container shall be marked with the following information:
- Delivery system
Table 1. Performance Parameters to Verify Safe and Reliable Deployment
Design Outputs
A complete specification and details of the whole technology system should be considered, including component and assembly-level specifications, delivery system, implant, accessories, packaging, and labeling including IFU and physician training material. Procedural steps and imaging guidance also are very important component of all mitral and tricuspid valve technologies.
Design Transfer
It is important to provide a clear and detailed flowchart identifying manufacturing process operation and inspection steps and indicating the input of all components and important manufacturing materials. It is also important to establish a control process necessary to ensure that product is the exact item that was designed for intended use. The verification process is necessary to demonstrate the acceptability of the process ranges chosen.
Risk Management
Based on ISO-14971, a risk management program should be defined and implemented, beginning with early stages of the development of a new technology.
Structural Integrity
The frame, or supporting structural component, is critical to the development of transcatheter-based therapies. Structural assessment should start with a stress analysis of the device. Steps in the analysis might include:
- Assessment of residual stresses
- Evaluation of use conditions to define inputs (boundary conditions) for modeling and simulation activities to assess applied stresses or strains
- Benchtop testing at appropriate physiologic loads
- Determination of constitutive relationships
- Validated stress analysis to understand magnitude and location of peak stress/strain values
Stress analysis is the basis upon which structural strategy assessment is developed. The early focus should be directed at assessing risk and identifying risk mitigation strategies. Key elements will be device fatigue and corrosion, and the following factors are important:
- The manufacturer should understand the fatigue endurance limit of the materials used in the design.
- Component level fatigue studies may be conducted to supplement the material fatigue analysis.
- Damage tolerant fatigue methodology may be used in lieu of component fatigue.
- Appropriate corrosion studies may be performed as defined by risk assessment (may include pitting/crevice corrosion, uniform/general corrosion, stress, galvanic and/or fretting corrosion)
Functional Performance
Key areas to consider with respect to functional performance should include the following:
- Basic implant structural properties and compatibility with the design and performance expectations.
- Crimping the implant into the delivery system and tracking the device through the anatomy can have an impact on the device performance. Crimp studies should be designed to understand any impact on the implant.
- Durability and wear testing strategy should be focused on demonstrating adequate durability to address significant safety concerns and support basic device functionality. The durability test strategy should be comprehensive enough to determine the wear characteristics and failure mode(s) of the device.
- Impact of preservation techniques on valve tissue
- Fixation chemistry
- Mechanical methods applied during chemical fixation
- Testing techniques to assess biomechanics
- Uniaxial
- Biaxial
- Tensile stress/strain and bending properties
- Crimp
- Characterize the condition of valve leaflets, skirt, and attachment after loading, tracking, and deployment. In the clinical setting, crimp duration can vary therefore these studies should consider worst case crimp durations.
- All testing for valve performance should be conducted on devices which have been crimped prior to testing.
- Wear
- These studies should be conducted to assess the wear characteristics of the device as compared to a clinically known control. An appropriate number of cycles may be required based on risk. Regardless of the duration of study the test environment must be carefully designed and executed in order to provide accurate and meaningful results.
- Assessments visually examined until failure include:
- Macroscopic damage
- Abrasion, holes, tears, delamination, fraying, coaptation, and dehiscence
- Accelerated Wear testing is an extremely valuable assessment but is very dependent on proper setup and monitoring
- Proper setting of the test system to achieve proper motion (i.e., full opening and closing). Setting the test system to achieve complete opening is critical. Setting a system such that a full opening is not achieved will lead to an inaccurate assessment of the wear characteristics.
- Accounting for tissue valve variations in correctly balancing the test system for all samples.
- Matching valves to a tester using the effective orifice area data from pulsatile flow testing will help in balancing of the system. High speed video imaging on a pulse duplicator under more physiologic conditions can be used to compare and adjust the wear test system. Use of a test system that allows for control of each valve on a tester is optimum.
- Overpressure: Excessively high closed valve pressure spikes can cause extreme damage and may mask the true wear characteristics. This can occur due to lack of system compliance, lack of adequate monitoring, poor system set up. This test is to evaluate wear that is representative of the clinical experience, not to intentionally destroy the valve and possibly mask true failure modes.
- The control group serves not only as a comparison to the test group, but it also serves as a control for the test system setup. A well-established database for the type and extent of wear expected is essential to make a valid comparison to the test group.
7. Failure mode analysis
-
- Some level of failure mode testing to provide an assessment of the ultimate failure mode(s). These tests should identify the weakest link in the device and may give insight into the robustness of the design that may not be seen through the accelerated wear studies.
- Failure modes seen during tests should concur with valve finite element analysis (FEA) models.
These areas of device characterization and testing represent the basic needed understanding of device deliverability, structural integrity, and function over the expected device life. This successfully completed testing package is taken alongside preclinical animal (in vivo) testing, described in the following section, to arrive at preparedness for consideration for human clinical testing. The bench top data as well as data retrieved from animal studies are expected to lower the risks of device use to arrive at an acceptable risk versus benefit profile for patients.
Design Validation (Preclinical) Studies
ISO 5840-3:2013 requires evaluation of the safety and performance of the new transcatheter technologies in a biological environment with the closest practical and feasible similarity to the human anatomy and condition. This final in vivo assessment, prior to human implantation, should provide an appropriate level of assurance about safety in the interaction of the body with the transcatheter system. A risk-based approach is required to determine what kind of in vivo animal studies are necessary. Acute studies may be adequate for assessment of established safe materials; however, using a new material requires chronic studies. In addition, acute studies may be good enough for assessment of delivery systems.
Although ovine and porcine hearts, which are the most common models for heart valve studies, are very similar to the human, there are some differences that must be considered. Smaller left atrial height in a porcine heart usually does not provide enough height for an appropriate interatrial septal puncture. The acute angle between the inferior vena cava and the long axis of the heart in the animal models prevents an accurate and appropriate assessment of the delivery system. Also, assessment of procedural steps over a different anatomy may not be the best option; instead, cadaver studies and three-dimensional printed models may provide a better insight.
For the same reasons, in order to assess the biological responses and hemodynamic performance, a surgical implantation is sometimes required. Additionally, the difference in imaging quality and visualization in large animals may prevent a transcatheter procedure and the only choice would be a surgical implant.
Another factor to be considered is the physical characteristics of mitral and tricuspid leaflets, chords, and annulus that are different and need to be taken into account for the technologies to work directly on these components. For instance, retention force of an anchor on the human vivid heart could be very different from animal heart or cadaver heart. In addition, having a diseased animal model is not always possible, and most of the animal studies are done on normal animals. Their heart characteristics could be very different from human cases. For example, the presence of calcification, fibrosis, degenerative valve disease, and so on present different tissue characteristics. Presence of these factors may interfere with the performance of the implants that need to sit on leaflets, chords, or annulus. Some, but not all, of these factors could be controlled by screening during the first-in-human evaluation. One extreme example of difficult-to-control factors is the thin tissue in Barlow’s disease. In this disease, which may involve both mitral and tricuspid valves, the characteristics of the connective tissue are very different from the normal heart.
Finally, the duration of the study must be used to drive the choice of preclinical animal model. If using a juvenile or nonadult animal in a long-term (>90 days) study, the growth rate of the animal needs to be taken into consideration, as the animal could outgrow the device implanted, affecting both fit and function of the device over time. In general, studies longer than 90 days should rely on adult animals not expected to increase in size after a certain age. What is considered the best option with one technology cannot be assumed with another.
Before any human implantation these tests are highly recommended:
- System-level Risk Assessment
- Critical hazards identified and sufficiently mitigated
- Risk-benefit reviewed and approved for the target population
- Structural Component Stress Analysis (FEA)
- Mechanical properties and boundary conditions justified
- Validated FEA under simulated use conditions
- Structural Component Fatigue Testing
- Structural Component Reliability Assessment
- Device Corrosion Assessment
- Pitting/crevice corrosion resistance characterized via testing
- Implant Durability
- Implant Hydrodynamic Performance
- Performance characterized under expected patient hemodynamic parameters and implant conditions
- Migration Resistance/Anchoring Integrity/Detachment Threshold
- Performance characterized to severe enough pressures for target implant conditions
- Usability and Deliverability Assessment
- Demonstrate that the device can be loaded and safely implanted by intended clinical investigators
- Preclinical In Vivo Evaluation (Chronic Animal Study)
- 90- to 140-day animal study in orthotopic implant position to evaluate:
- Delivery, deployment, implantation, and imaging characteristics
- Hemodynamic performance
- In vivo response/compatibility
- 90- to 140-day animal study in orthotopic implant position to evaluate:
- Crimp Damage Assessments
- Characterize the condition of all components, and attachment after loading, tracking, and deployment
Manufacturing
Upon successful completion of the design process, and validation of the design via clinical study, the manufacturer must demonstrate the capability of producing the device repeatedly within the required specifications. A significant undertaking, this is done to ensure that the mass-produced devices deliver the results observed during design validation. Validation of the individual manufacturing processes requires an evaluation of those critical processes that drive the functional performance of the devices, and demonstration that those processes are stable and show repeated conformance to specifications. In general, the activities are divided into installation, operational, and performance qualifications.
— Installation qualifications – Performed to document objective evidence that all equipment used within a process has been installed correctly, and all functions are operating as intended.
— Operational qualifications – Performed to document evidence that a process, when performed at its extreme high/low parameters, produces a product that meets the required process outputs. This evaluates the robustness of the process.
— Performance qualification – Performed to document evidence that a process can repeatedly produce a product which meets required outputs consistently over multiple lots/batches.
Manufacturing validations are performed as per the above as a requirement for commercial distribution of the device upon regulatory approval. These activities can often take as long as the design process and require a great deal of planning and resources for execution. Additionally, these activities may not be completed when a device is being studied in human clinical trials, and a manufacture may rely on verification of the outputs of each lot/batch produced, rather than investing time in the validation of a process that could change in response to validation data or further design changes. Some activities within the manufacturing overall process are identified as highly critical (e.g., sterilization) and may require continuous monitoring even after the process has been validated. Further, all manufacturing validation activities are also subject to the manufacturer’s quality management system, and an updated level of documentation must be maintained.
The following standards are all relevant to provide guidance for the process described above:
| Document # | Title |
| EN ISO 5840-1:2015 | Cardiovascular implants – Cardiac valve prostheses Part 1: General requirements |
| ISO 5840-3:2013 | Cardiovascular implants – Cardiac valve prostheses Part 3: Heart Valve Substitutes Implanted by Transcatheter Techniques |
| EN ISO 14630: 2012 | Non-active surgical implants – General requirements (ISO 14630:2012) |
| EN ISO 25539-1:2009 | Cardiovascular implants – endovascular devices – Part 1: Endovascular prostheses (ISO 25539-1:2003 including Amd 1:2005) |
| ASTM D4169-14 | Standard Practice for Performance Testing of Shipping Containers and Systems |
| ASTM E112-13 | Standard Test Methods for Determining Average Grain Size |
| ASTM F2182-11a | Standard Test Method for Measurement of Radio Frequency Induced Heating On or Near Passive Implants During Magnetic Resonance Imaging |
| ASTM F2213-06 | Standard Test Method for Measurement of a Magnetically Induced Torque on Medical Devices in the Magnetic Resonance Environment |
| ASTM F2052-15 | Standard Test Method for Measurement of a Magnetically Induced Displacement Force on Medical Devices in the Magnetic Resonance Environment |
| ASTM F2119-07 | Standard Test Method for Evaluation of MR Image Artifacts from Passive Implants |
| ASTM F2503-13 | Standard Practice for Marking Medical Devices and Other Items for Safety in the Magnetic Resonance Environment |
| EN 556-1:2001 | Sterilization of medical devices – Requirements for medical devices to be designated “STERILE” – Part 1: Requirements for terminally sterilized medical devices (incorporating Corr. 1, 2006) |
| ISO 15223-1:2012 | Medical devices – Symbols to be used with medical device labels, labelling and information to be supplied – Part 1: General requirements |
| EN 1041:2008+A1 2013 | Information supplied by the manufacturer of medical devices |
| EN ISO 14971: 2012 | Medical devices – Application of risk management to medical devices (ISO 14971:2007) |
| EN ISO 13485: 2012 | Medical devices – Quality management systems- Requirements for regulatory purposes |
| EN ISO 14155:2011 | Clinical investigation of medical devices for human subjects – Good Clinical Practice (ISO 14155:2011) |
| ISO 14644-1:2015 | Clean rooms and associated controlled environments – Part 1 – Classification of air cleanliness |
| EN ISO 14644-2:2015 | Clean rooms and associated controlled environments – Part 2 – Specifications for testing and monitoring to prove continued compliance with ISO 14644-1 |
| EN ISO 10993-1:2009
(Apply in part) |
Biological evaluation of medical devices – Part 1: Evaluation and testing within a risk management process (ISO 10993-1:2009 and Corrigendum June 2010) |
| EN ISO 10993-3:2014 | Biological evaluation of medical devices – Part 3: Tests for genotoxicity, carcinogenicity, and reproductive toxicity (ISO 10993-3:2014) |
| EN ISO 10993-4:2009 | Biological evaluation of medical devices – Part 4: Selection of tests for interactions with blood (ISO 10993-4:2002, including Amd 1:2006) |
| EN ISO 10993-5:2009 | Biological evaluation of medical devices – Part 5: Tests for cytotoxicity |
| EN ISO 11607-1:2009+A1:2014 | Packaging for terminally sterilized medical devices – Part 1: Requirements for materials, sterile barrier systems and packaging systems (ISO 11607-1:2006) |
| EN ISO 10993-6:2009 | Biological evaluation of medical devices – Part 6: Tests for local effects after implantation (ISO 10993-6:2007) |
| EN ISO 10993-10:2013 | Biological evaluation of medical devices – Part 10: Tests for irritation and skin sensitization (ISO 10993-10:2010) |
| EN ISO 10993-11:2009 | Biological evaluation of medical devices – Part 11: Tests for systemic toxicity (ISO 10993-11:2006) |
| EN ISO 11737-1:2006 | Sterilization of medical devices—Microbiological methods—Part 1: Determination of a population of microorganisms on products (ISO 11737-1:2006) |
| EN ISO 11135:2014 | Sterilization of health-care products – Ethylene oxide – Requirements for the development, validation and routine control of a sterilization process for medical devices (ISO 11135:2014) |
| EN ISO 11137-1:2015 | Sterilization of health care products – Radiation Part 1: Requirements for development, validation and routine control of a sterilization process for medical devices (ISO 11137-1:2006 including Amd 1:2013) |
| EN ISO 11137-2:2015 | Sterilization of health care products – Radiation Part 2: Establishing the sterilization dose (ISO 11137-2:2013) |
| ASTM F720-13 | Standard Practice for Testing Guinea Pigs for Contact Allergens: Guinea Pig Maximization Test |
| ASTM F2063-12 | Standard Specification for Wrought Nickel-Titanium Shape Memory Alloys for Medical Devices and Surgical Implants |
| ASTM F2082-15 | Standard Test Method for Determination of Transformation Temperature of Nickel-Titanium Shape Memory Alloys by Bend and Free Recovery |
| ASTM E8/E8M-15a | Standard Test Methods for Tension Testing of Metallic Materials |
| ASTM F2129-15 | Standard Test Method for Conducting Cyclic Potentiodynamic Polarization Measurements to Determine the Corrosion Susceptibility of Small Implant Devices |
